Loading
Loading
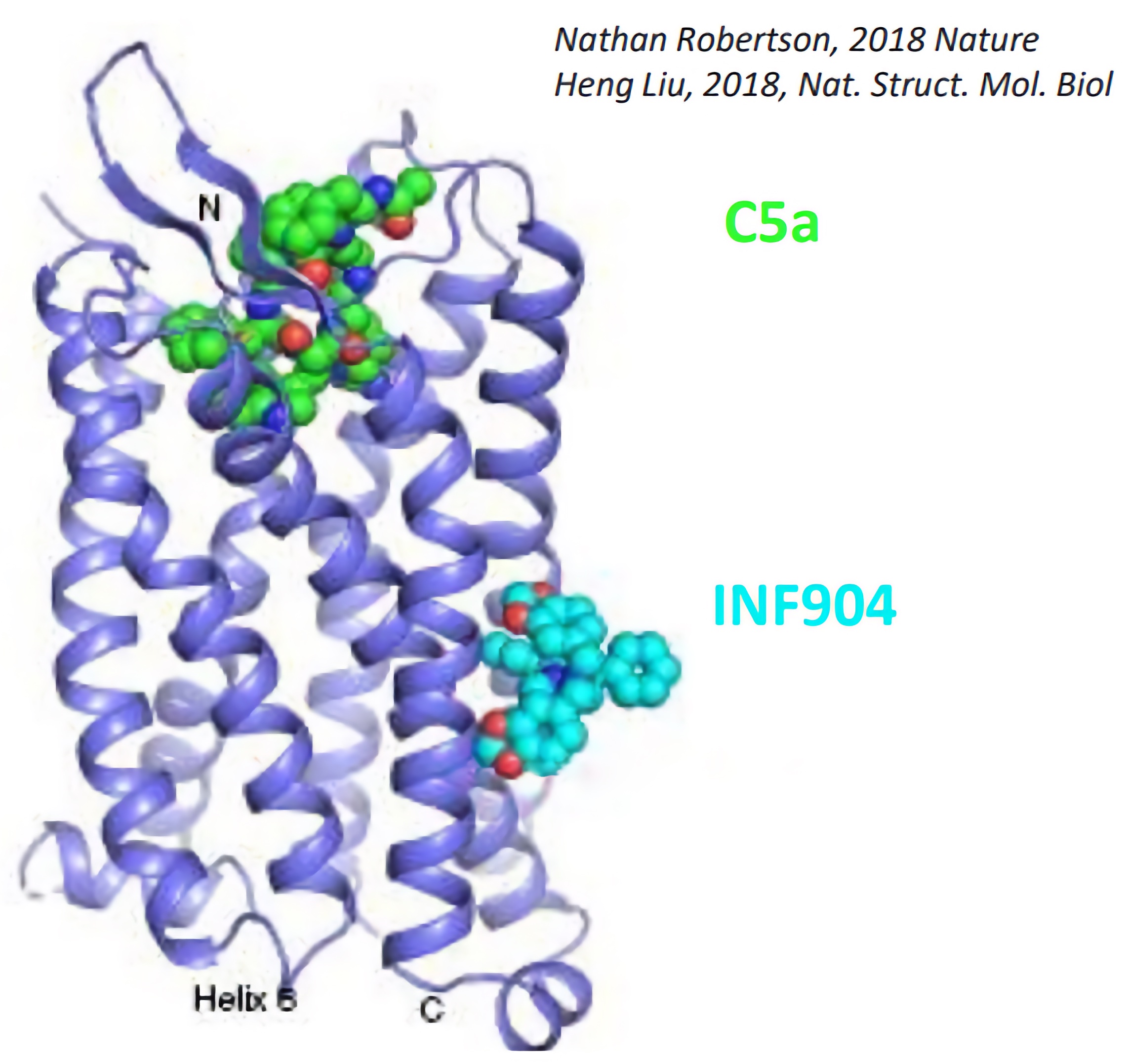
INF904 is a potentially best-in-class oral, low molecular weight drug that targets C5aR with high affinity and selectivity; and binds to an allosteric site on C5aR1 within the lipid bilayer, antagonizing the binding of C5a to block induction of neutrophil activation. As a “pipeline-in-a-product” InflaRx is addressing complement-mediated, immune and inflammatory conditions with INF904.
After completing Phase 2a trials in chronic spontaneous urticaria (CSU) and hidradenitis suppurativa (HS), InflaRx intends to broaden development further into HS and CSU, and into areas such as nephrology, neurology and hematology via potential future collaborations with partners. The Company continues to conduct required additional supportive studies with INF904 as well.
In preclinical studies, INF904 showed higher plasma exposure in animals, including non-human primates, and improved inhibitory activity in a hamster neutropenia model compared to the marketed C5aR inhibitor. Further, in contrast to the marketed C5aR inhibitor, in vitro experiments demonstrated that INF904 has minimal inhibition of the cytochrome P450 3A4/5 (CYP3A4/5) enzymes, which play an important role in the metabolism of a variety of metabolites and drugs, including glucocorticoids. Anti-inflammatory therapeutic effects in several preclinical disease models were also demonstrated by INF904 as well.
Reported results from a first-in-human study demonstrated that INF904 has best-in-class potential, is well tolerated in treated subjects and exhibits no safety signals of concern in single doses ranging from 3 mg to 240 mg or multiple doses ranging from 30 mg once per day (QD) to 90 mg twice per day (BID) for 14 days. Pharmacokinetic / pharmacodynamic data showed INF904 provided ≥90% blockade of C5a-induced neutrophil activation achieved over the 14-day dosing period. In addition, compared to the marketed C5aR inhibitor, INF904 showed ~3-fold higher Cmax and ~10-fold higher AUClast (at comparable dosing levels) and faster achievement of therapeutic exposures with a broad therapeutic index (at BID and QD dosing).
InflaRx has pursued two initial immuno-dermatology indications with INF904, with data reported in HS and CSU in November 2025. The trial is a multi-center, open-label study in patients with moderate-to-severe CSU and moderate-to-severe HS and evaluated multiple INF904 dosing regimens over 4 weeks of treatment (with 4 additional weeks of follow up without dosing). The primary goals were to generate additional safety and pharmacokinetic (PK) data and to provide signs of clinical benefit.
In the HS, across all dosing groups, INF904 induced rapid, meaningful and consistent reductions in ANs and dTs; in addition to improvements in measures such as HiSCR, IHS4, NRS30, and DLQI. Improvements in reported efficacy measures were largely rapid and consistent, be-ginning from Week 1, and deepened over the 4-week treatment period. All doses showed positive clinical activity and appeared strongest in the highest dosing cohort. The company believes efficacy data for INF904 at 4 weeks were largely in line with data reported for the same timepoint from clinical studies for approved HS therapies, and compared favorably for reductions in dT and improvement in NRS30 response.
Furthermore, initial data reported from 25 HS patients that completed the 4-week off-drug follow-up period showed that HiSCR responses continued to deepen 4 weeks after the treatment period. Preliminary PK results from Week 8 indicate INF904 levels 4 weeks off drug may still offer C5aR blocking potential. In addition, InflaRx believes inhibiting C5aR may drive an improvement in the inflammatory environment which may confer longer lasting benefit.
Safety data from 33 HS patients was reported. No signal of safety concern was detected, with no reported serious adverse events (SAEs) and three adverse events in two patients reported as possibly-to-likely related to drug which all were mild (Grade 1) in nature.
Overall, InflaRx believes these data are strong evidence that INF904 is highly active in HS, with improvements in efficacy measures largely differentiated from historically reported placebo, and in line with reported improvements at the 4-week time point for therapies which have successfully completed Phase 3 trials or received approval.
In CSU, reported improvements in clinical measures such as UAS7 appeared generally higher in the 60 mg dosing cohort (UAS7 change from baseline of -13.7 points at Week 4) and indicate a level of activity that exceeds average historically reported placebo levels, and is within the range of existing approved CSU therapies. Furthermore, in patients with severe CSU at baseline (UAS7 of 28–42, n=23), the 60mg dose reduced UAS7 by 15.4 points, and in patients who presented with angioedema (n=3) the reduction of UAS7 was 18.7 points. Additional efficacy analyses in patients with high IgE (n=22) and low IgE (n=6) at baseline showed that INF904 appeared equally effective in both patient populations.
Furthermore, initial data reported from 23 CSU patients that completed the 4-week off-drug observational follow-up period, indicated that patients continued to benefit from INF904 four weeks after the last dose, with responses in the 60mg dosing cohort showing a mean absolute UAS7 reduction of 16.7. InflaRx believes that INF904’s ability to drive durable improvements within the inflammatory environment could provide deepened clinical benefit with long-term dosing.
Safety data from 33 CSU patients was reported. No signal of safety concern was detected, with no reported SAEs and one adverse event reported as possibly related to drug which was mild (Grade 1) in nature.
Overall, InflaRx believes these data suggest that INF904 is active in CSU and not only differentiated from reported historical placebo rates, but potentially within the range of currently approved CSU therapies. These data further indicate that UAS7 decreases may deepen with longer-term treatment.