
Jena, Germany, 6 November 2019 – InflaRx (Nasdaq: IFRX), a clinical-stage biopharmaceutical company developing anti-inflammatory therapeutics by targeting the complement system, today reported results of the open label extension (OLE) part of the international SHINE Phase IIb study investigating the safety and efficacy of IFX-1, a first-in-class anti-human complement factor C5a monoclonal antibody, in patients with moderate to severe Hidradenitis Suppurativa (HS), a painful and debilitating chronic inflammatory skin disease with limited treatment options. The data announced today are from a snapshot analysis at the end of the overall 9-month study treatment period (week 40).
A total of 156 patients entered the 6-month OLE period upon completion of week 16 of the recently reported SHINE study. The SHINE study was a Phase IIb randomized, double-blind, placebo-controlled, multicenter trial which enrolled a total of 179 patients with moderate to severe HS in four active dose arms of IFX-1 (minimal, low, medium and high dose) and a placebo arm. The main trial did not meet its primary endpoint of a dose dependent drug effect on Hidradenitis Suppurativa Clinical Response Score (HiSCR)¹
Patients participating in the OLE part of the study remained blinded to their initial treatment regimen and were grouped into two arms, responders and non-responders, according to the HiSCR at week 16. The Responder Group received a maintenance IFX-1 treatment dose of 800 mg every 4 weeks to investigate if they would maintain their response. The Non-responder Group received an IFX-1 treatment of 800 mg every 2 weeks to investigate if they would become responders. As induction therapy, patients transitioning from the former minimal dose or placebo groups received one or two additional 800 mg infusions, respectively.
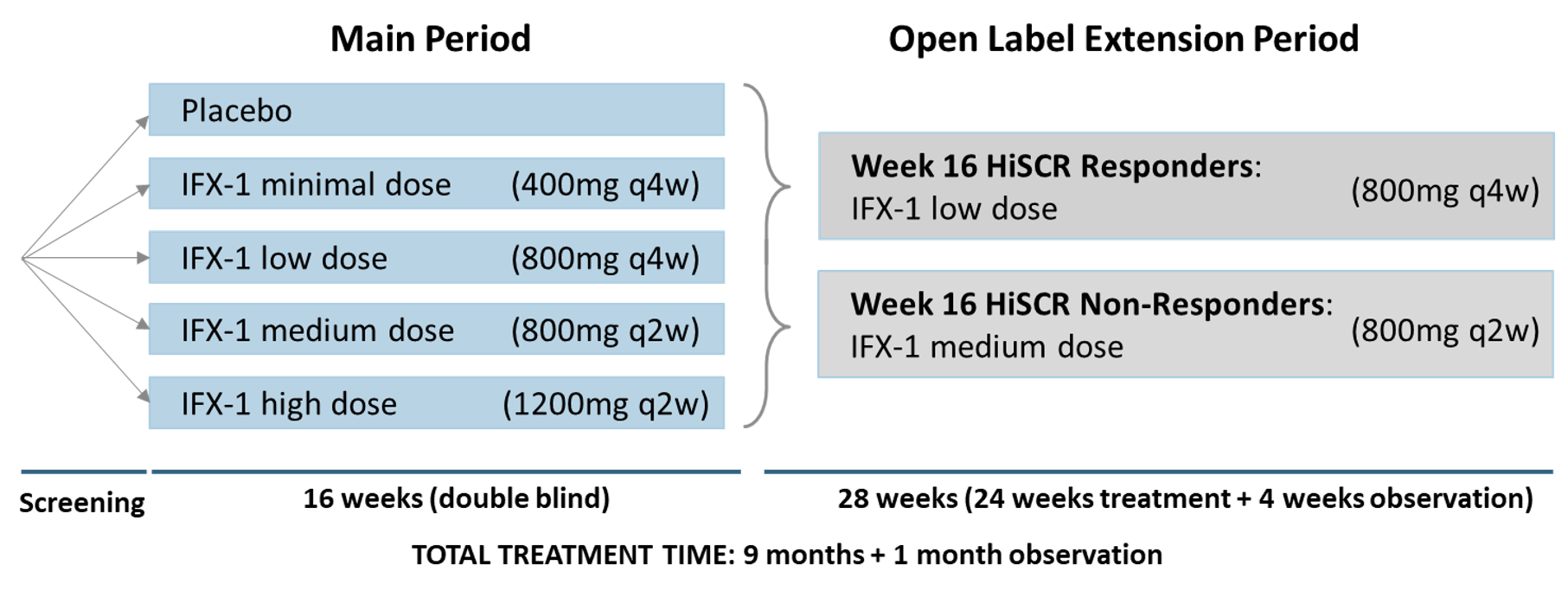
Figure 1: Trial Design Scheme
Of the 156 patients entering the OLE part, 72 were in the Responder Group and 84 in the Non-responder Group. A total of 122 of the 156 patients completed the OLE phase (78.2 %) - 93.1% (n=67) of the Responder Group and 65.5% (n=55) of the Non-responder Group.
Response according to HiSCR
The endpoint focus for the OLE part of the study was HiSCR response rate at week 40:
Thus, at the end of the 9-month treatment period, 56.3% of all patients who completed the OLE were HiSCR responders.
Improvements in patients completing the OLE treatment period at week 40 (all patients)
Overall, patients completing the OLE period showed a sustained improvement in inflammatory lesion count at week 40 compared to baseline counts of the OLE treatment group on day 1 of the SHINE study. There was a relative reduction in the total body count of:
These results were also reflected in the International Hidradenitis Suppurativa Severity Score System (IHS-4)². The IHS-4 score for these patients improved markedly, with a relative change of -54.5% (mean) and -64.1% (median) when compared to the day 1 baseline values of the OLE patient group.
Results related to the Responder Group and Non-responder Group
Similar to the results detected for the HiSCR, improvements in inflammatory lesion counts and the IHS-4 scores in the Responder Group (on the lower 800 mg IFX-1 every 4 week dose) were largely maintained or slightly decreased throughout the OLE treatment period, while the Non-responder Group (on the biweekly 800 mg IFX-1 dosing) consistently showed an overall improvement throughout the OLE treatment period. The improvements in the Non-responder Group were especially pronounced in those patients who were in the former placebo and minimal dose groups in the main period of the study.
Christopher Sayed, MD, Associate Professor of Dermatology, University of North Carolina School of Medicine, Chapel Hill, NC, USA commented: “The data from the open label extension part of the SHINE study demonstrate an impressive reduction of inflammatory lesions in patients completing the overall 9-months treatment period. Given that close to 70% of patients who started treatment in the SHINE trial completed the extension part, a considerable percentage of patients experienced a long-term improvement while being treated with IFX-1.”
Prof. Dr. med. Evangelos J. Giamarellos-Bourboulis, ATTIKON University Hospital in Athens, Greece, the principal investigator of this trial, commented: “I am happy to see that the data from the long-term extension phase of the SHINE study confirm the long-term durable effects and the inflammatory lesion reduction detected in the original Phase IIa study which we conducted”.
IFX-1 continues to be well tolerated over long term
Long-term treatment with IFX-1 was well tolerated. No drug-related serious adverse events (SAEs) were reported during the OLE part of the trial (total of 9 SAEs unrelated to treatment reported in 5 patients). No correlation between adverse events and IFX-1 dose levels could be detected.
Prof. Niels C. Riedemann, CEO and Founder of InflaRx, commented: “The data from the open label extension part of the SHINE study are very encouraging and confirm our conclusions from the recently published post-hoc analysis that IFX-1 may hold a large potential to help patients suffering from HS. We will now evaluate a path forward for the development of IFX-1 in HS, which includes planned discussions with regulatory agencies.”
The Company has conducted in-depth pharmacokinetic and pharmacodynamic analyses and related modeling which indicate a good tissue penetration of IFX-1 and a target (C5a) mediated drug clearance. The IFX-1 consumption rate in HS patients was considerably higher when compared to IFX-1 plasma levels in other diseases, likely caused by a very high C5a turnover rate in HS. This explains the need for a high drug dose of IXF-1 as supported by the findings of the recently published post-hoc analysis.
InflaRx plans to discuss the SHINE study data with regulatory authorities and to submit the study results for publication in a peer-reviewed medical journal upon completion of the data base lock and final analysis.
Strategic update call scheduled for 7 November 2019
The Company will host a conference call to provide details on the study and a strategic update in conjunction with its quarterly results on 7 November at 8am EST / 2pm CET.
Dial-in details:
From the US: +1 929 477 0402
From the UK: +44 (0) 330 336 9127
From Europe: +49 (0) 69 2222 25577
Conference Code: 3567762
Please dial in 10 minutes before the call to register. The webcast presentation for the conference call can be accessed here.
About IFX-1:
IFX-1 is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity towards its target in human blood. Thus, IFX-1 leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism, which is not the case for molecules blocking the cleavage of C5. IFX-1 has been demonstrated to control the inflammatory response driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response in pre-clinical studies. IFX-1 is believed to be the first monoclonal anti-C5a antibody introduced into clinical development. Approximately 300 people have been treated with IFX-1 in clinical trials, and the antibody has been shown to be well tolerated. IFX-1 is currently being developed for various inflammatory indications, including Hidradenitis Suppurativa, ANCA-associated vasculitis and Pyoderma Gangraenosum.
About InflaRx N.V.:
InflaRx (Nasdaq: IFRX) is a clinical-stage biopharmaceutical company focused on applying its proprietary anti-C5a technology to discover and develop first-in-class, potent and specific inhibitors of C5a. Complement C5a is a powerful inflammatory mediator involved in the progression of a wide variety of autoimmune and other inflammatory diseases. InflaRx was founded in 2007 and the group has offices and subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI, USA. For further information, please visit www.inflarx.com.
Contacts:
InflaRx N.V.
Prof. Dr. Niels C. Riedemann, CEO
info[at]inflarx.de
+49-3641-508180
Investor / Media Relations Support
MC Services AG
Katja Arnold, Laurie Doyle, Andreas Jungfer
Europe: +49 89-210 2280
US: +1-339-832-0752
¹ To be considered a responder according to the HiSCR, the total body count of abscesses and inflammatory nodules (AN count) must be reduced by at least 50% and the abscesses and draining fistula count may not exceed baseline counts. The score does not take into account reduction in draining fistulas.
² International Hidradenitis Suppurativa Severity Score System (IHS-4): Scores the patient according to all inflammatory lesions, in contrast to the HiSCR. In this compounded score, each inflammatory nodule is counted with 1 point, each abscess with 2 points and each draining fistula with 4 points.
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “estimate,” “predict,” “potential” or “continue” and similar expressions. Forward-looking statements appear in a number of places throughout this release and may include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our intellectual property position, our ability to develop commercial functions, expectations regarding clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, future transactions, growth and strategies, the industry in which we operate, the trends that may affect the industry or us and the risks uncertainties and other factors described under the heading “Risk Factors” in InflaRx’s periodic filings with the Securities and Exchange Commission. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.